OSMOSIS
● The `color{violet}("plant cell")` is surrounded by a `color{brown}("cell membrane")` and a `color{brown}("cell wall.")`
● The cell wall is `color{brown}("freely permeable")` to water and substances in solution hence is not a barrier to movement.
● In `color{violet}("plants the cells")` usually contain a large `color{brown}("central vacuole")`, whose contents, the `color{brown}("vacuolar sap")`, contribute to the `color{brown}("solute potential")` of the cell.
● In `color{violet}("plant cells,")` the cell membrane and the membrane of the vacuole, the `color{brown}("tonoplast")` together are important determinants of `color{violet}("movement of molecules")` in or out of the cell.
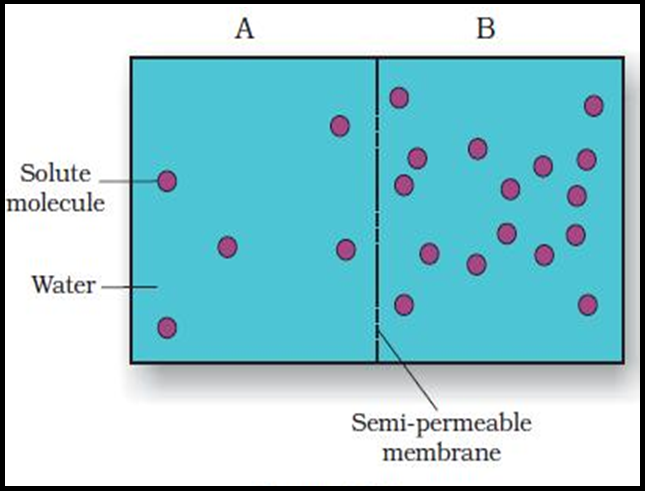
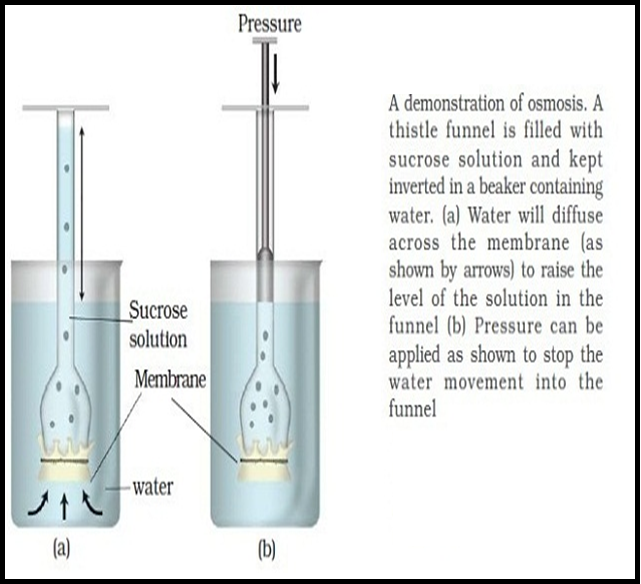
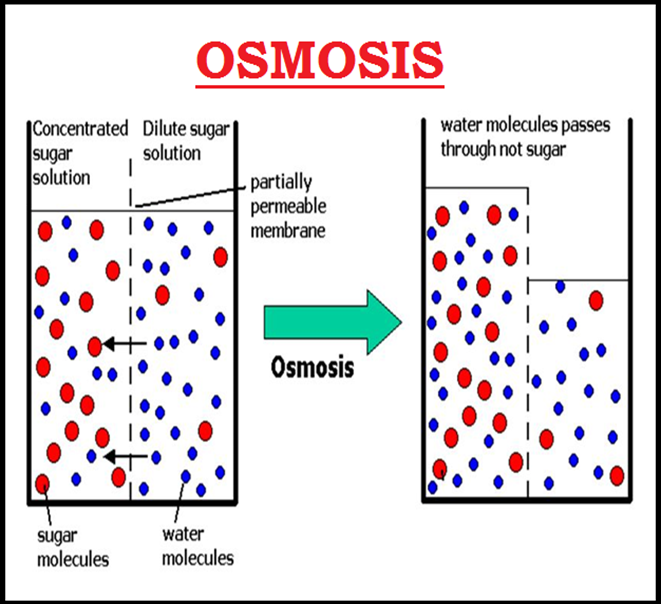
● `color{brown}("Osmosis")` is the term used to refer specifically to the `color{violet}("diffusion of water")` across a differentially- or `color{brown}("semi-permeable membrane.")`
● `color{violet}("Osmosis")` occurs spontaneously in response to a `color{brown}("driving force.")`
● The net direction and rate of osmosis depends on both the `color{brown}("pressure gradient")` and `color{brown}("concentration gradient.")`
● Water will move from its region of `color{brown}("higher chemical potential")` (or concentration) to its region of lower chemical potential until equilibrium is reached.
● At equilibrium the two chambers should have the `color{violet}("same water potential.")`
● The cell wall is `color{brown}("freely permeable")` to water and substances in solution hence is not a barrier to movement.
● In `color{violet}("plants the cells")` usually contain a large `color{brown}("central vacuole")`, whose contents, the `color{brown}("vacuolar sap")`, contribute to the `color{brown}("solute potential")` of the cell.
● In `color{violet}("plant cells,")` the cell membrane and the membrane of the vacuole, the `color{brown}("tonoplast")` together are important determinants of `color{violet}("movement of molecules")` in or out of the cell.
● `color{brown}("Osmosis")` is the term used to refer specifically to the `color{violet}("diffusion of water")` across a differentially- or `color{brown}("semi-permeable membrane.")`
● `color{violet}("Osmosis")` occurs spontaneously in response to a `color{brown}("driving force.")`
● The net direction and rate of osmosis depends on both the `color{brown}("pressure gradient")` and `color{brown}("concentration gradient.")`
● Water will move from its region of `color{brown}("higher chemical potential")` (or concentration) to its region of lower chemical potential until equilibrium is reached.
● At equilibrium the two chambers should have the `color{violet}("same water potential.")`